HIV VACCINES
Unmet medical needs in HIV/AIDS Therapy - Focus on South Africa (RSA)
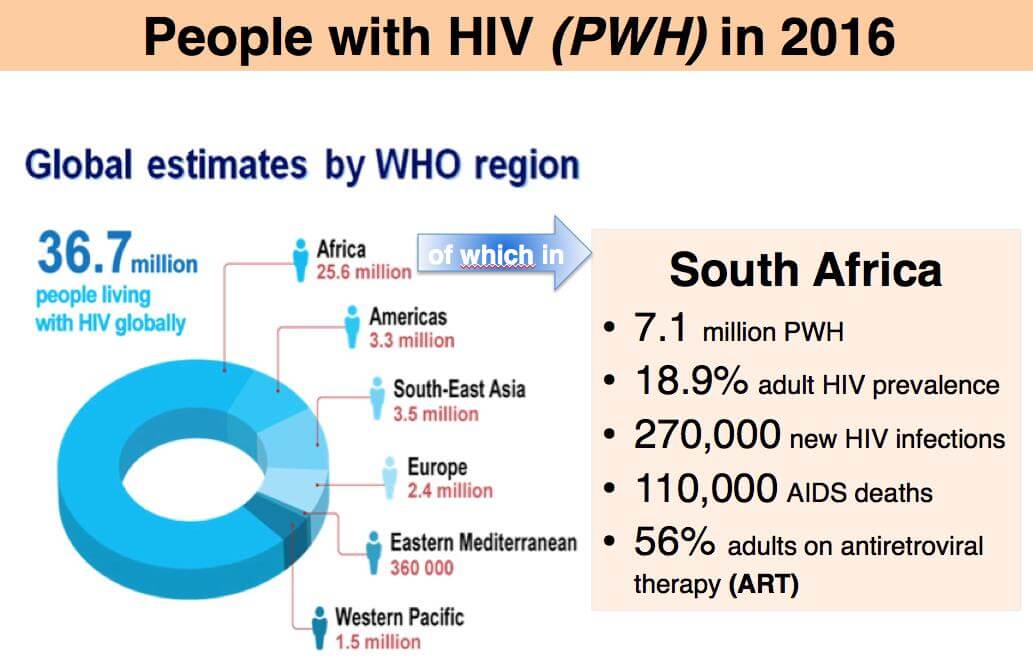
Nearly 40 million people in the world live with HIV (PH+), of which 27 million in Sub-Saharan Africa where, in spite of antiretroviral therapy (ART), the infection is increasing especially among women and young people, the most exposed to the virus. Women are hardest hit accounting for the majority of people infected.
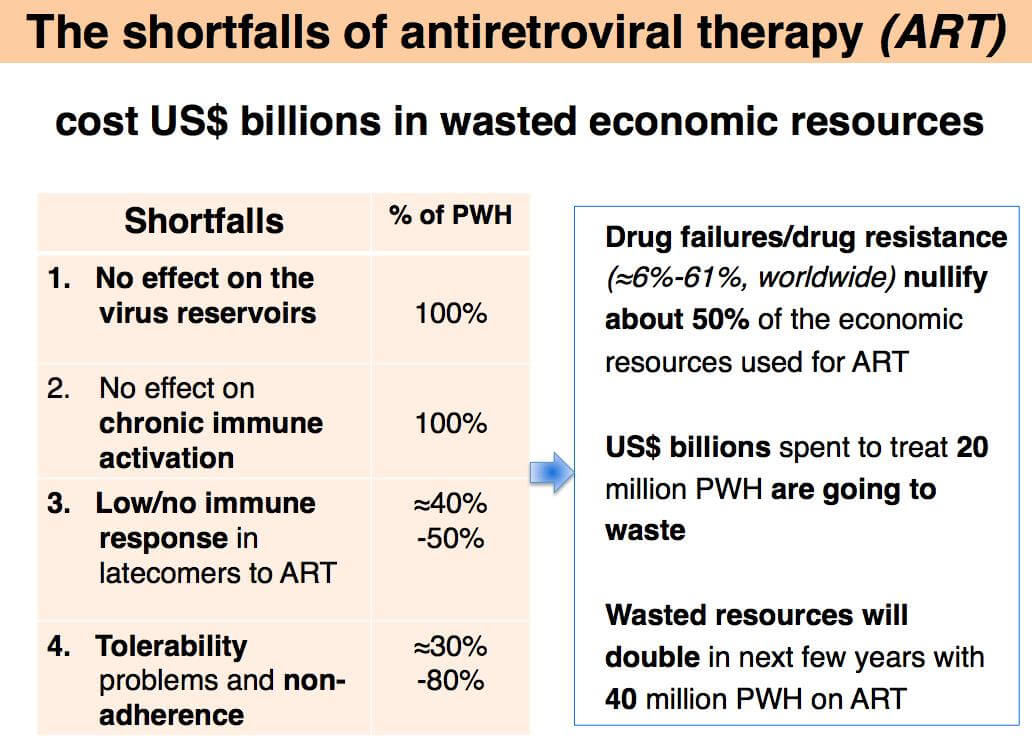
South Africa has the world’s largest epidemic, 7.1 million PH+, 20% of the population. New infections (>300.000/year) continue in spite of massive ART programs (2017: 4 million PH+, US$ 2 billion spent for ART) that are being jeopardized by serious therapeutic shortfalls of ART:
TATIMMUNE® - A novel therapeutic vaccine for PH+ on ART
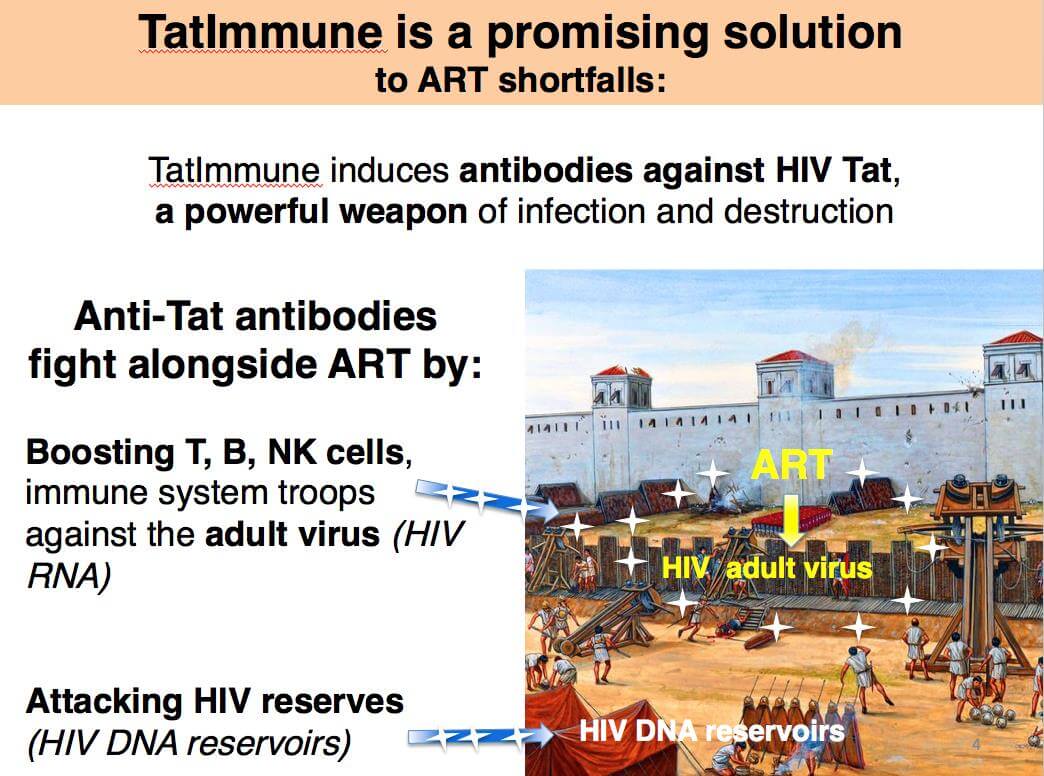
TatImmune represents a promising solution to ART shortfalls, compensating where ART fails by targeting HIV-1 Tat, a key virulence factor essential for virus replication and infectivity, and, importantly, for the virus reservoir against which ART has no effect. TatImmune is given once in a lifetime: 3 doses, one month apart.
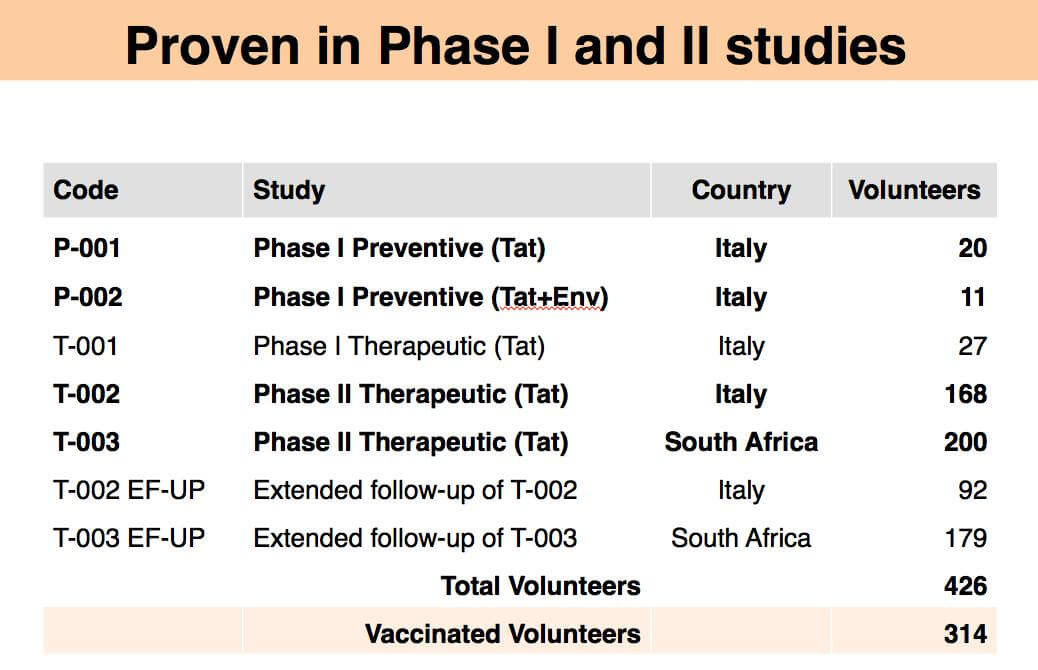
Clinically tested in 3 phase I studies (Italy), and 2 phase II studies in Italy and RSA, with 314 volunteers of different race, sex, ART regimen and HIV virus subtype. Results show strong durable boost of immune defences (CD4/CD8 T cells, B and NK cells) and drastic durable reduction of virus reservoirs against which ART has no effect (-70% after 8 years). Efficacy demonstrated with major types of ART regimens and HIV subtypes.
TatImmune was invented and has been developed by Dr Barbara Ensoli, Director of the National HIV/AIDS Research Center of the Italian National Institute of Health and former member of the Scientific Council of the European Research Council.
Funding for clinical trials (about EUR 30 Million over 15 years) was provided by Italian and European research grants. The results are published and have been independently assessed by the South Africa National Department of Health and by UNIDO, the United Nations Industrial Development Organization, both advocating registration and use in South Africa.
Phase III licensure studies in RSA will enrol recently diagnosed PH+ and PH+ on ART for many years, aiming to improve response to ART in the former, and enhancing/intensifying ART efficacy in the latter.
Trial protocols have already been discussed (Sept. 2017) with SAHPRA, the regulatory body of South Africa, and will be discussed with the US Food and Drug Administration. The South African trials will support licensure studies in the USA and Europe.